-
1. Review the targeted good practice measure(s) criteria and evidence required sections. Be as confident as you can that your product(s) meets the requirements.
-
2. Ensure all the required evidence is collected in electronic versions and can be submitted with the application.
-
3. Complete the application form and submit with all required evidence.
-
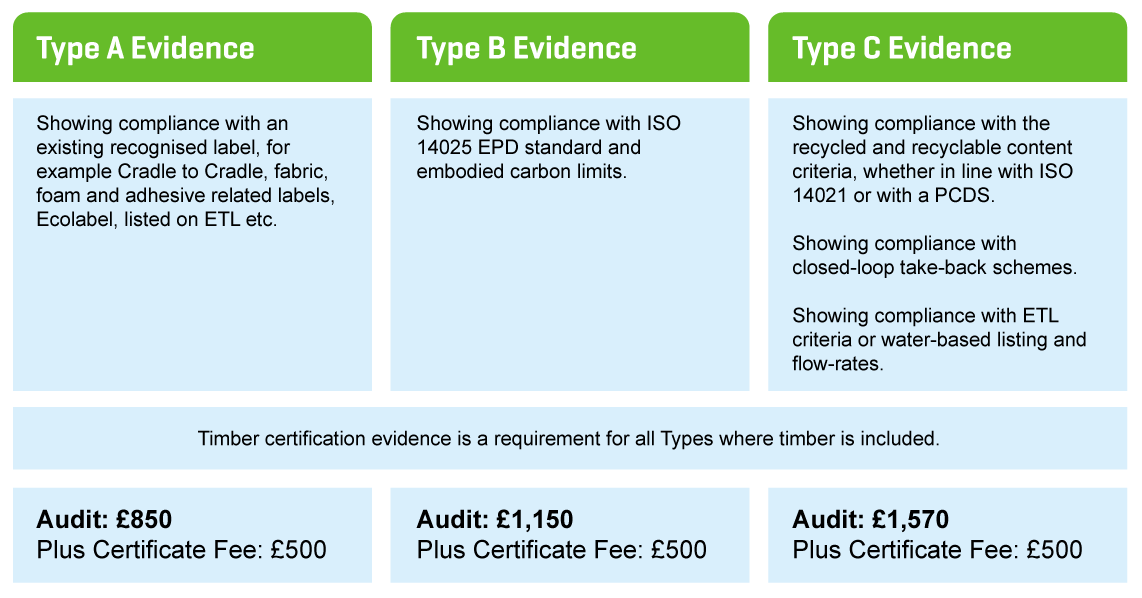
4. Make payment based on the type of evidence you are submitting (A, B or C) for each application.
-
5. Following receipt of payment(s), evidence submitted is cheked for completeness, and once all is received, confirmation of the audit commencement is provided.
-
6. The review is undertaken within 3 weeks of the commencement date and the result(s) are communicated to you. If non-compliant is the outcome we share feedback on reasons, and if it is compliant then we start the certification and contract process.
-
7. Once the label licence is signed by a responsible party of the applicant, we issue the label asset and certificate(s) and add the Compliant products to the published Product Directory. This directory is updated monthly as products are certified.